Additional information
| Active substance | Esomeprazole |
|---|---|
| Acne | No |
| Water Retention | No |
| HBR | No |
| Hepatotoxicity | NO |
| Aromatization | No |
| Lab Test | Not specific |
| Also known as | Esomeprazole magnesium |
| WAREHOUSE | International Warehouse 2 |
| Blood pressure | No significant effect |
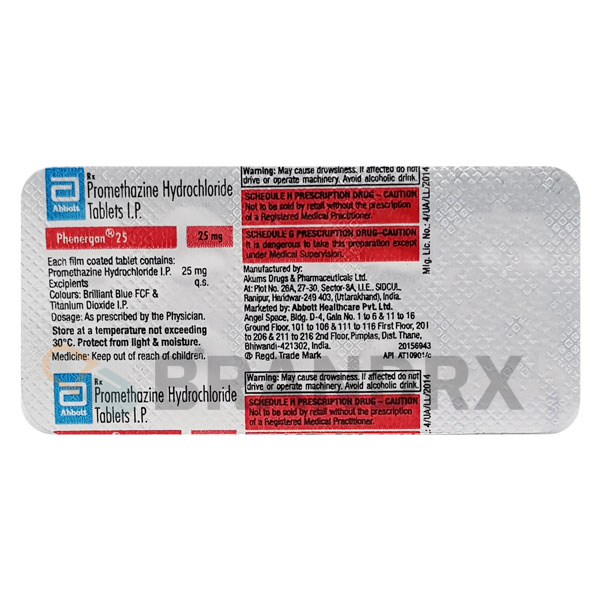
| Trade name | Nexium |
| Storage conditions | Store at room temperature, keep away from moisture and heat |
| Chemical name | (S)-5-Methoxy-2[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole |
| Formula | C17H19N3O3S |
| Substance class | Proton pump inhibitor |
| Main action | Reduces stomach acid production by blocking the enzyme system of gastric parietal cells |
| Half-life | 1-1.5 hours |
| Dosage (medical) | 20-40 mg daily |
| Dosage (sports) | Not applicable |
| Effects | Decreases gastric acid secretion, increases gastric pH |
| Side effects | Headache, diarrhea, nausea, flatulence, abdominal pain, constipation, dry mouth |
| Use in sports | Not typically used |
| Manufacturer | AstraZeneca |




Reviews
There are no reviews yet.